Slippery findings about impure ice
As winter approaches in the Northern hemisphere, many will soon face one of their toughest foes: ice. From delaying flights to making roads slippery, ice accumulation on surfaces wreaks havoc in many ways.
But not all ice is created equal. In new research from the University of Illinois at Chicago (UIC), scientists studied the stickiness of ice containing everyday contaminants such as salt, soap and alcohol. They report their findings in a paper in Materials Horizons.
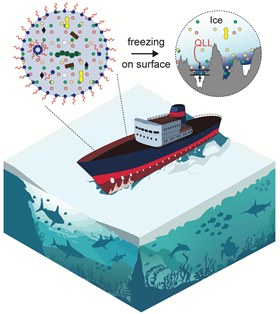
Most laboratory studies typically test ice made from pure water, but in nature, ice is seldom pure. “Be it dirty sidewalks or the hull of Arctic-going marine ships, there’s always impurities there,” said Sushant Anand, associate professor of mechanical and industrial engineering at UIC and senior author of the paper. “So, the natural question that comes to mind is: what is the influence of these compounds on how strongly ice sticks to surfaces?”
Anand’s laboratory prepared ice with varying concentrations of contaminants and tested how strongly they clung to different industrial materials. Surprisingly, they found that, under certain conditions, impure ice was much less sticky than ice made from pure water.
They traced the cause of this slipperiness back to the way water freezes when it contains impurities and the unique structure that forms when ice touches a solid material, called a quasi-liquid layer. “The ice region near a solid has liquid-like properties, and its thickness could contribute to how tightly ice sticks,” Anand said. “But this region is really difficult to analyze through experiments.”
So, he teamed up with UIC colleague Subramanian Sankaranarayanan and his group at UIC and Argonne National Laboratory to study this layer and how it changes with different levels of impurities, using molecular dynamics simulations. They found that as impure water freezes, it expels contaminants that drain along channels and ice-grain boundaries toward the ice base, where it forms a liquid layer that gives the ice extra slipperiness.
“These insights could lead to the design of next-generation winterization techniques that slowly release contaminants to promote facile ice shedding,” said graduated PhD student Rukmava Chatterjee, who is the first author of the paper.
These surprising test results raise another question: if small salt concentrations make ice less likely to stick to surfaces, why do ships in arctic climates that sail through salt water still struggle with ice formation?
Experiments revealed that the water freezing rate can influence how impurities migrate to regions where ice touches a solid. A slow freezing process causes the isolation of contaminants into concentrated pockets or even their complete expulsion, producing purer and stronger ice. Faster freezing preserves the contaminants within the ice and their accumulation at the ice-solid interface, leading to weaker adhesion.
“Our study represents just the tip of the iceberg, opening new lines of investigation of how impure ice adheres, with widespread implications across multiple disciplines,” Anand said.
This story is adapted from material from the University of Illinois at Chicago, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.
Source link



